
Food


CBD brands apply for certification as Google lifts ads ban in some parts of US
10th February 2023 - News analysis |
Cannabis companies are looking to get Google certification as part of the tech giant’s update on the use of Google Ads for CBD products in parts of the US

Greece: CBD and cannabis regulation, February 2023
3rd February 2023 - Regulatory reports |
This report provides a detailed overview of the current regulatory framework in place for CBD and other cannabinoids, hemp and cannabis

EFSA denies waiting to follow US FDA’s lead on CBD safety in novel foods
1st February 2023 - News analysis |
The European Food Safety Authority denies it is waiting research results on the safety of CBD by the US Food and Drug Administration before it lets validated candidates complete the novel food application process

22nd Century Group acquires a UK cannabinoid distributor for $650K
30th January 2023 - News analysis |
US company 22nd Century Group has acquired privately held UK cannabinoid distributor RX Pharmatech for an up-front payment of $650,000 in cash and stock

US FDA says lack of evidence for dietary-supplement pathway to CBD regulation
26th January 2023 - News analysis |
The US Food and Drug Administration (FDA) has rejected a regulatory pathway that would allow CBD products to be treated as food additives or dietary supplements

Russia: CBD and cannabis regulation, January 2023
19th January 2023 - Regulatory reports |
This report provides a detailed overview of the current regulatory framework in place in Russia for hemp, CBD and cannabis, covering all areas of policy

Denmark: CBD and cannabis regulation, January 2023
17th January 2023 - Regulatory reports |
This report offers a comprehensive overview of the current regulatory framework in place in Denmark for hemp, CBD and other cannabinoids, as well as medical and recreational cannabis

Belgium: CBD and cannabis regulation, January 2023
16th January 2023 - Regulatory reports |
This report provides a detailed overview of Belgium’s federal and state regulation of hemp, CBD and cannabis, covering everything from cultivation to extracts and finished products, as well as the medical and recreational cannabis regimes

FDA expected to issue recommendation ‘soon’ on CBD regulation in the US
13th January 2023 - News analysis |
The US Food and Drug Administration (FDA) may soon issue recommendations for a long-awaited regulation of CBD products in the country

Monaco: CBD and cannabis regulation, January 2023
9th January 2023 - Regulatory reports |
This report provides a detailed summary of the current regulations in place for hemp, cannabis and CBD in Monaco, covering all areas from cultivation and processing to extracts and finished products

Australia: CBD and cannabis regulation, January 2023
4th January 2023 - Regulatory reports |
This report provides a detailed overview of Australia’s federal and state regulation of hemp, CBD and cannabis, covering everything from cultivation to extracts and finished products, as well as the medical and recreational cannabis regimes

US FDA again issues warning letters on CBD in ingestibles, this time with a twist
30th December 2022 - News analysis |
The FDA has made clear for years that CBD in food is illegal in the US, as it violates the Federal Food, Drug, and Cosmetic (FD&C) Act. What’s different this time?

Italy: CBD and cannabis regulation, December 2022
19th December 2022 - Regulatory reports |
This report provides a detailed overview of the current regulatory framework in place for CBD, hemp and cannabis, covering all policy areas

Glasses raised to Canada’s relaxation of the purchasing limit on cannabis drinks
15th December 2022 - News analysis |
Canada’s cannabis industry is toasting the federal government’s announcement of new regulations that will dramatically increase the number of cannabis drinks consumers can buy at one time

Germany: CBD and cannabis snapshot
15th December 2022 - Market reports , Regulatory reports |
This snapshot of Germany’s CBD and cannabis sector covers both the regulatory framework for CBD as well as cannabis (recreational and medical) and offers an overview of the market, online retailers and consumer preferences

Spain: CBD and cannabis snapshot
14th December 2022 - Market reports , Regulatory reports |
This snapshot of Spain’s CBD and cannabis sector covers both the regulatory framework for CBD as well as cannabis (recreational and medical) and offers an overview of the market, online retailers and consumer preferences

Simon the brand-builder demonstrates confidence in future of cannabis drinks
7th December 2022 - Blogs |
Cannabis-infused drinks have been the subject of industry debate and speculation as they raise many challenges – from regulatory hurdles to dosage issues and taste formulation – but is it just a case of “when”, not “if”?

South Korea: CBD and cannabis regulation, November 2022
30th November 2022 - Regulatory reports |
The South Korean legislation does not make a clear distinction between hemp and cannabis. This report provides a detailed overview of the regulatory framework in place for hemp, cannabinoids and cannabis, covering all policy areas

Netherlands: CBD and cannabis snapshot
30th November 2022 - Market reports , Regulatory reports |
This snapshot of the Netherland’s CBD and cannabis sector covers both the regulatory framework for CBD as well as cannabis (recreational and medical) and offers an overview of the market, online retailers and consumer preferences

France: CBD and cannabis snapshot
29th November 2022 - Market reports , Regulatory reports |
This snapshot of France’s CBD and cannabis sector covers both the regulatory framework for CBD as well as cannabis (recreational and medical) and offers an overview of the market, online retailers and consumer preferences

Tilray bets on cannabis-infused drinks, grows distribution network in the US
25th November 2022 - News analysis |
Tilray Brands continues to show signs of confidence in the future of cannabis-infused drinks by adding more beverage firms to its portfolio and building a distribution network

Lebanon: CBD and cannabis regulation, November 2022
24th November 2022 - Regulatory reports |
This report provides a detailed overview of the current regulatory framework in place in Lebanon for hemp, cannabinoids such as CBD, and cannabis

UK FSA novel food process can succeed with ‘supportive and sensible approach’
21st November 2022 - News analysis |
A cannabis regulation lawyer believes the UK Food Standards Agency (FSA) and the CBD sector have invested so much time, effort and money in the novel food process that it cannot be allowed to fail

EIHA’s ‘reasonable daily intake’ sows confusion over novel food applications
17th November 2022 - News analysis |
CBD isolate producers in the UK are waiting to see whether and how toxicological data released by the European Industrial Hemp Association (EIHA) will affect their novel food applications to the Food Standards Agency (FSA)

More validated but no light at the end of the tunnel for Efsa’s novel food applicants
16th November 2022 - News analysis |
Nearly six months after the European Food Safety Authority (Efsa) put novel food assessments for cannabinoids on hold, none of the validated applicants has been able to fill the identified knowledge gaps

No UK novel food authorisations likely before mid-2023 – and no guarantees
10th November 2022 - News analysis |
It’s unlikely that any applications for novel food authorisation of CBD products in the UK will be considered for authorisation before mid-2023 – and being on the list of validated applications is no guarantee

Amazon cannabinoid prohibition policy not strictly enforced on UK, US sites
28th October 2022 - News analysis |
Amazon still seems to be laissez-faire about enforcement of ingestible hemp-derived cannabinoid products that appear on its site – and in particular those containing intoxicating hemp-derived cannabinoids (IHDCs)

Croatian cannabis and hemp business owners feel the ‘unfair’ weight of the law
30th September 2022 - News analysis |
A Croatian hemp producer is challenging the interpretation used by law enforcement to oppose the sale of hemp flower in the country

French hemp industry trade association wants consortium to fill EFSA’s data gap
21st September 2022 - News analysis |
A French hemp trade association believes the consortium approach applied in the initial European novel food application process could again be the solution to the requests for extra data that have stalled assessments

CBD brands hit supermarket shelves as UK market shows mainstream appeal
14th September 2022 - News analysis |
Despite the challenges of the market, CBD firms appear to be setting their sights on the UK and have secured some major listings in mainstream supermarkets

France: CBD and cannabis regulation, September 2022
14th September 2022 - Regulatory reports |
This report provides a detailed summary of the current regulations in place for hemp, cannabis and CBD in France, covering all areas from cultivation and processing to extracts and finished products

Morocco: CBD and cannabis regulation, September 2022
2nd September 2022 - Regulatory reports |
This report provides a detailed overview of the regulatory framework in place in Morocco for hemp, cannabis and CBD

China: CBD and cannabis regulation, September 2022
1st September 2022 - Regulatory reports |
Cannabis and cannabis extracts are controlled substances under national legislation in China. This report provides a detailed overview of national and local regulation of hemp, CBD and cannabis

Hong Kong: CBD and cannabis regulation, August 2022
24th August 2022 - Regulatory reports |
This report provides a detailed overview of the regulatory framework in place in Hong Kong for hemp, cannabis and CBD

Swiss CBD company takes issue with EFSA’s delay of novel food applications
1st August 2022 - News analysis |
A Swiss CBD company has questioned the approach of European authorities in demanding further data in order to advance novel food applications, describing it as an issue that might signal the end of hemp-extracted cannabinoids as a viable sector in the EU

UK: CBD and cannabis snapshot
18th July 2022 - Market reports , Regulatory reports |
This snapshot of the UK’s CBD and cannabis sector covers both the regulatory framework for CBD as well as cannabis (recreational and medical) and offers an overview of the market, online retailers and consumer preferences

Austria: CBD and cannabis regulation, July 2022
7th July 2022 - Regulatory reports |
This report offers a detailed summary of the current regulatory framework in place for CBD, hemp and cannabis in Austria. It covers everything from hemp cultivation and processing, to finished products and extracts, and the country’s medical and recreational cannabis policies
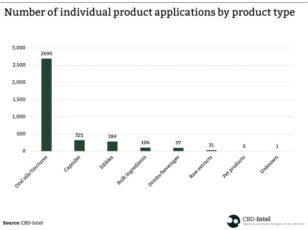
CBD edible oils dominate the UK’s novel food product applications
6th July 2022 - Regulatory reports |
On 31st March 2022, exactly one year after the novel food application deadline for CBD food products in the UK, the UK Food Standards Agency (FSA) published a list of around 3,500 CBD food products whose applications for novel food status have been validated or still await validation

Why novel food rules are pushing the UK market towards cold-pressed products
4th July 2022 - News analysis |
Companies are facing technical and marketing pros and cons as they increasingly turn to cold-press CBD extraction as a way around novel food market limitations in the UK and Ireland

No magic solution: EFSA ingestible CBD safety concerns won’t be resolved quickly
1st July 2022 - Blogs , News analysis |
It looks like there will be no easy or quick solutions to addressing the data gaps the European Food Safety Authority (EFSA) requires to reach a conclusion on the safety of CBD as an ingestible ingredient

UK FSA issues final final list of products linked to a CBD novel food application
30th June 2022 - News analysis |
The UK Food Standards Agency (FSA) has published its final public list of CBD products that can be marketed in England and Wales – and again it’s twice as long as it was

EU novel food applications: the science behind what EFSA says it wants to know
27th June 2022 - News analysis |
The European Food Safety Authority wants more safety data before it approves CBD products as novel food. This is what it wants to know, and why, along with a summary of the science on each point

State AGs urge US Congress leaders to take action on ‘copycat’ THC edibles
24th June 2022 - News analysis |
A bipartisan group of 23 US state attorneys general have joined calls to halt the sale of edible cannabinoid-containing products that imitate the look of well-known brands

Why the long pause? Companies question Efsa’s insistence on filling ‘data gap’
23rd June 2022 - Blogs , News analysis |
In trying to be overly cautious on novel foods, has the European Food Safety Authority inadvertently added an extra risk to public health?

Data gaps in EU novel food applications will be difficult, slow and costly to fill
15th June 2022 - News analysis |
CBD companies say they do not know when or even how they will be able to provide the missing data that has caused the European Food Safety Authority (EFSA) to pause the clock on assessing novel food applications

EFSA puts novel food assessment on hold for applicants to provide missing data
7th June 2022 - News analysis |
A hold has been placed on European novel food assessment after the European Food Safety Authority (EFSA) said it was unable to proceed due to identified knowledge gaps in safety data

FDA sends warnings to companies selling CBD-containing veterinary products
1st June 2022 - News analysis |
The US Food and Drug Administration (FDA) has issued warnings to four companies that have sold veterinary products containing CBD

FSS confirms enforcement of non-compliant products…
27th May 2022 - Alerts | Europe, Scotland, United Kingdom |

UK FSA’s slow-moving novel food authorisation process feeds frustration
20th May 2022 - News analysis |
It’s been two years since the deadline for CBD companies to apply for the Food Standards Agency (FSA) novel foods authorisation, yet none have been authorised and few have been validated



